STUDY ON THE CORRELATION BETWEEN STRUCTURES OF BITHIAZOLE DERIVATIVES AND PLASMID DNA CLEAVAGE ACTIVITY BY USING SIMULATION AND EXPERIMENT
Tatsuya Takimoto, Hiroki Takahashi, Hirohito Tsue, Koji Aoki, Miho Yamamoto, Eori Okazaki, Tatsuki Betsuyaku and Hideaki Sasaki
Faculty of Pharmaceutical Sciences, Kobe Gakuin University, Kobe, Japan; Graduate School of Human and Environmental Studies, Kyoto University, Kobe, Japan
Abstract
We have reported that bis(aminoalkyl)bithiazoles 1, 2 (Fig. 1) show the strong activity of pBR322 plasmid DNA cleavage in the presence of specific metal ions [1, 2]. In order to study the relationship of the structure and metal ions, we focused their stable structure of the complexes those bithiazole derivatives and metal ions. Aiming to form stable complex bithiazole derivatives and metal ions, we synthesized 4,4’-bithiazolophanes with two short bridges 3, 4 (Fig. 1) by the reaction of thioamide and dibromoalkanediones with microwave irradiation and searched their activities in the presence of various metal ions. 3 showed the activities of plasmid DNA cleavage in the presence of Cu2+. However, 4 did not show the activity in the same condition. In order to discuss this metal ion selectivity, we simulated the structure of complex those bithiazole derivatives 1 – 4 and metal ions by using MOPAC 2002 (PM5 level) and estimated the most stable structure and their heat of formation energy of the complexes. In conclusion, it was found that bis(aminomethyl)bithiazole moiety complex with Cu2+, bis(aminoethyl)bithiazole moiety complex with Co2+ easily. In this conference, we will report the synthesis and X-ray crystallographic analysis of bithiazolophanes and simulated structure of the complex in detail, the correlation between structures of bithiazole derivatives 1 – 4 and their activities of plasmid DNA cleavage.Keywords: Bithiazoles, cyclophane, plasmid DNA cleavage, simulation, new drug, metal complex.
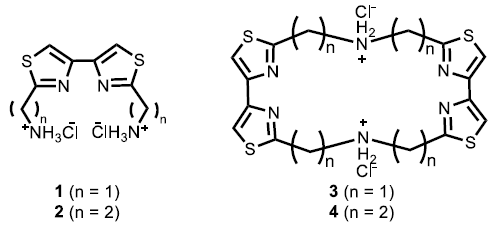
Fig. (1). Bis(2,2'-aminoalkyl)-4,4'-bithiazoles 1, 2 and 4,4’-bithiazolophanes 3, 4.
REFERENCES
[1] Sasaki, H., Tetrahedron Lett. 35(25), 1994, 4401.
[2] Sasaki, H., et al., Chem. Pharm. Bull. 44(9), 1996, 1761.